


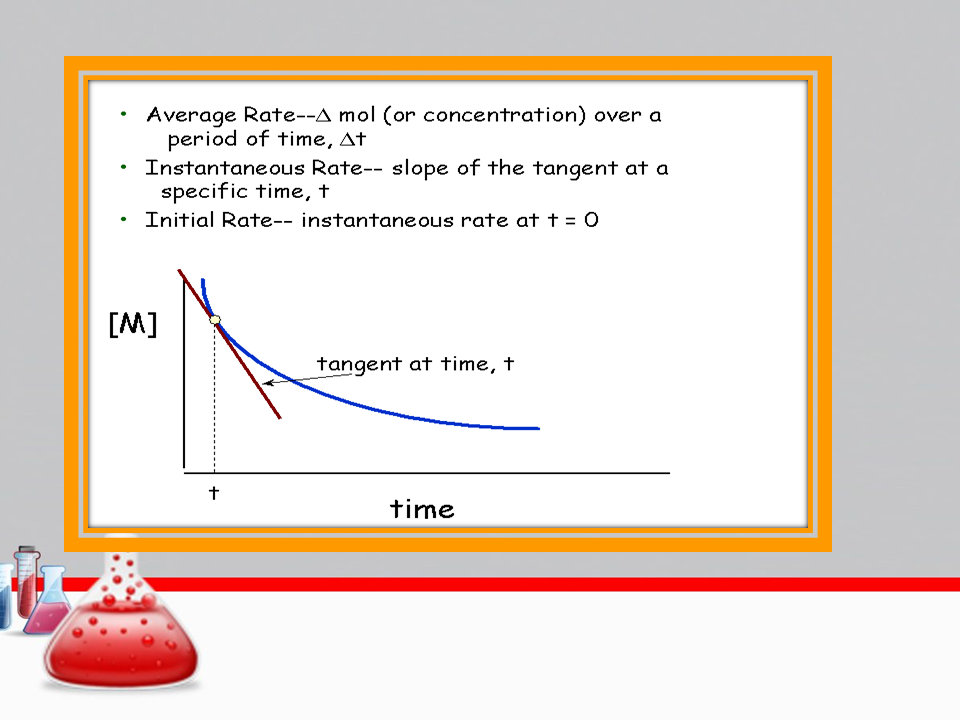
OBJECTIVE-Calculate half-life.
Deduce the order of a reaction.
12A / 12 B
|
16.03.2020
|

_________________________________________________________________________________
12A/12B |
12A-17.03.2020
|
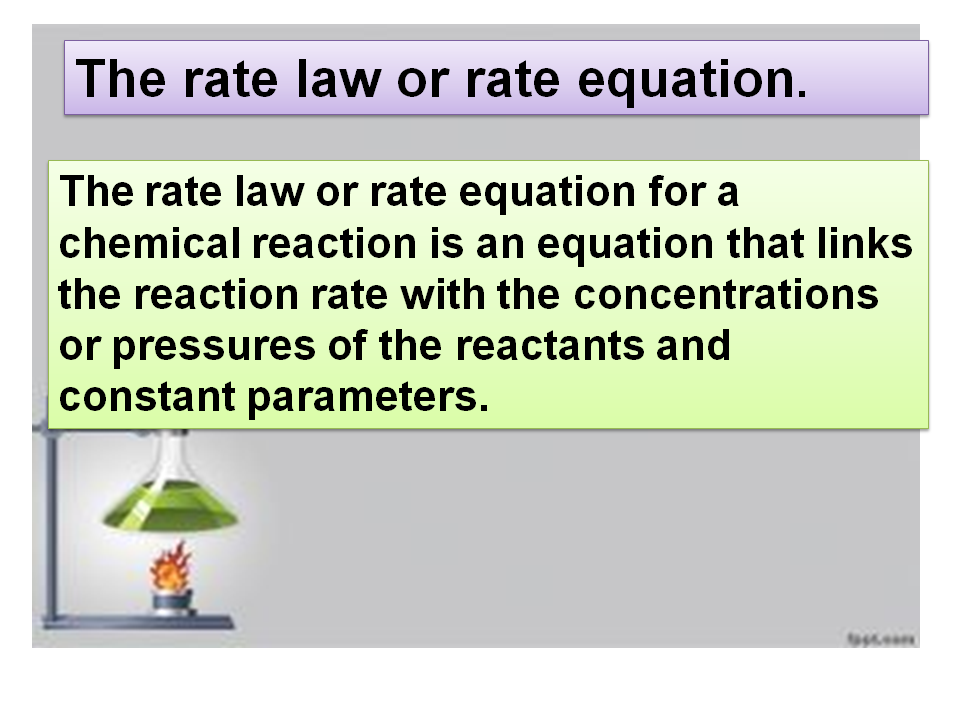
Unit- 2 chemical energetic.Deduce the order of a reaction. |
Presentation.
|




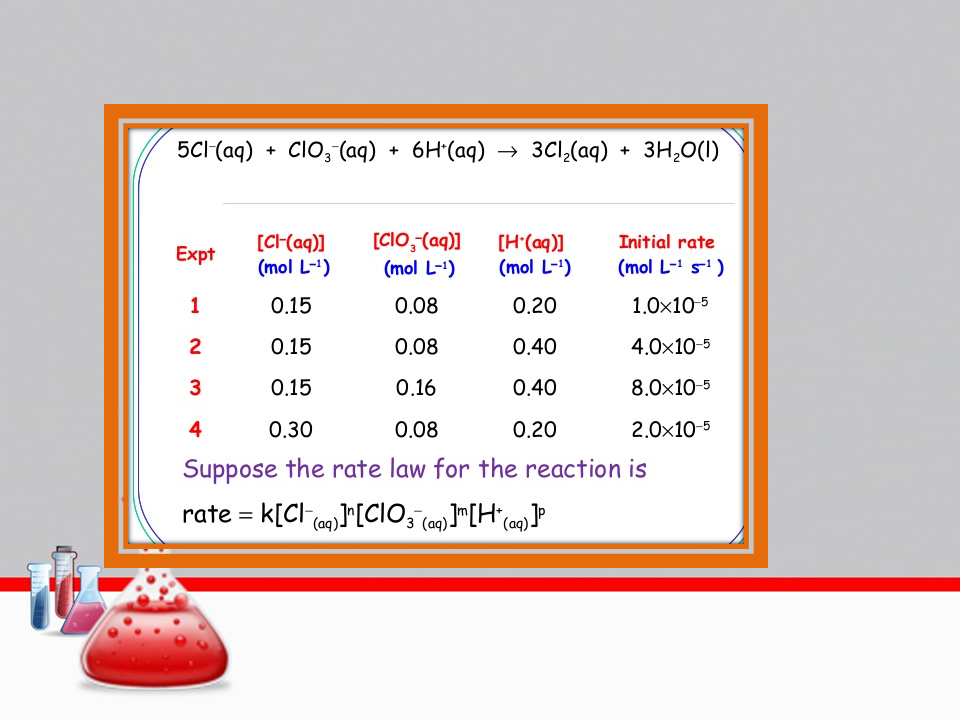
Using the following data, which is the correct
rate law of the sample reaction?
A + 5B + 6C → 3D + 3E
| Experiment | [A] (M) | [B] (M) | [C] (M) | Initial Rate (M/s) |
|---|---|---|---|---|
| 1 | 0.35 | 0.35 | 0.35 | 8.0 x 10start superscript, negative, 4, end superscript |
| 2 | 0.70 | 0.35 | 0.35 | 3.2 x 10start superscript, negative, 3, end superscript |
| 3 | 0.70 | 0.70 | 0.35 | 6.4 x 10start superscript, negative, 3, end superscript |
| 4 | 0.70 | 0.35 | 0.70 | 3.2 x 10 |
Determine the reaction order from the relative rate information.
| Relative [A] (M) | Relative Rate (M/s) |
|---|---|
| 1 | 1 |
| 2 | 1 |
| 3 | 1 |
https://www.youtube.com/watch?v=xGeXosOUeVE&list=PLX4e2DxFRGQJCol8PlXtxUaKmaj1XvXKD&index=4



























































































No comments:
Post a Comment