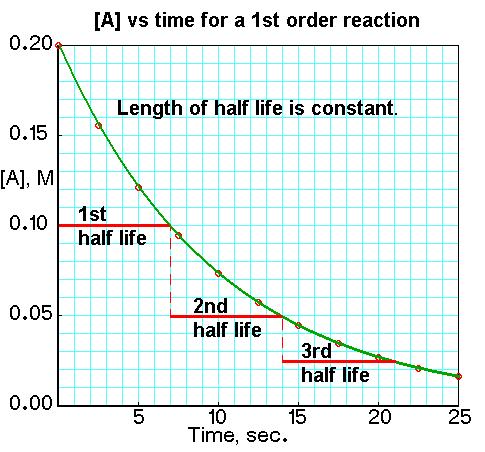
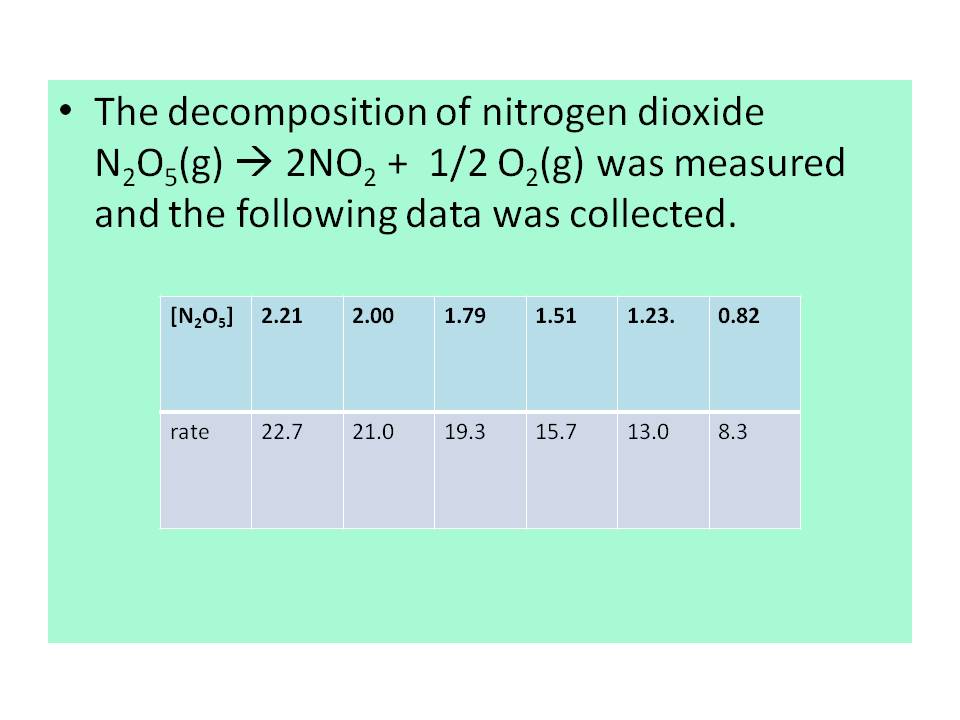
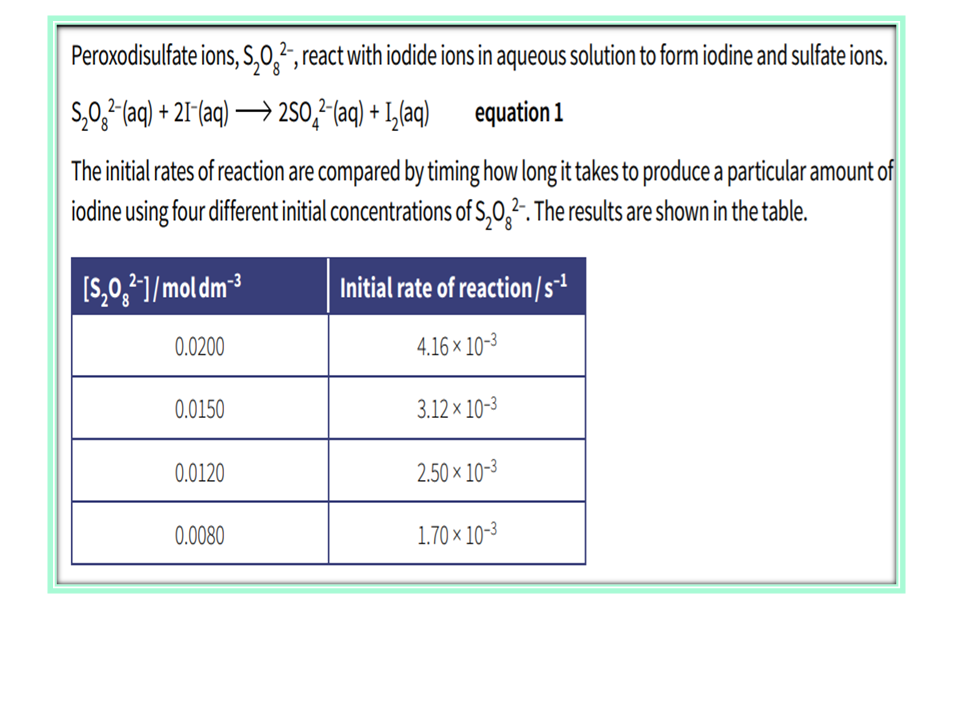
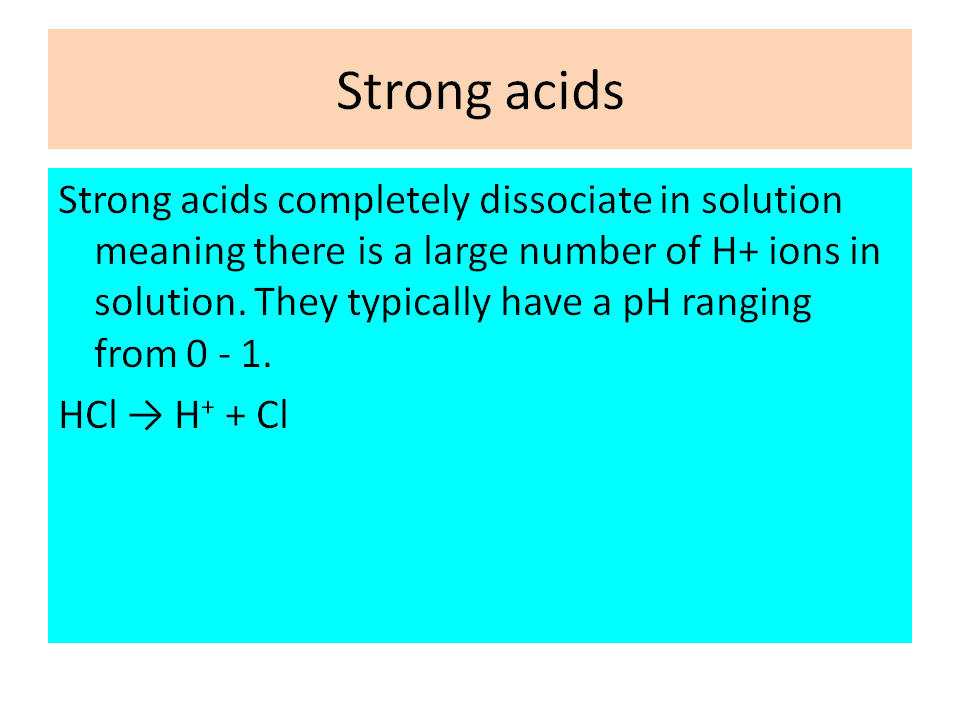
.
3.The
initial rate of the reaction between the gases NO and H2 was measured
in a series of experiments at a constant temperature and the following rate
equation was determined.
rate
= k[NO]2[H2]
(a)Complete the table of data below for the
reaction between NO and H2
Experiment
|
Initial
[NO] / mol dm–3
|
Initial
[H2] / mol dm–3
|
Initial
rate / mol dm–3 s–1
|
1
|
3.0 × 10–3 |
1.0 × 10–3 |
1.8 × 10–5 |
2 |
3.0 × 10–3 |
7.2 × 10–5 |
|
3 |
1.5 × 10–3 |
1.0 × 10–3 |
|
4 |
0.50 × 10–3 |
8.1 × 10–5 |
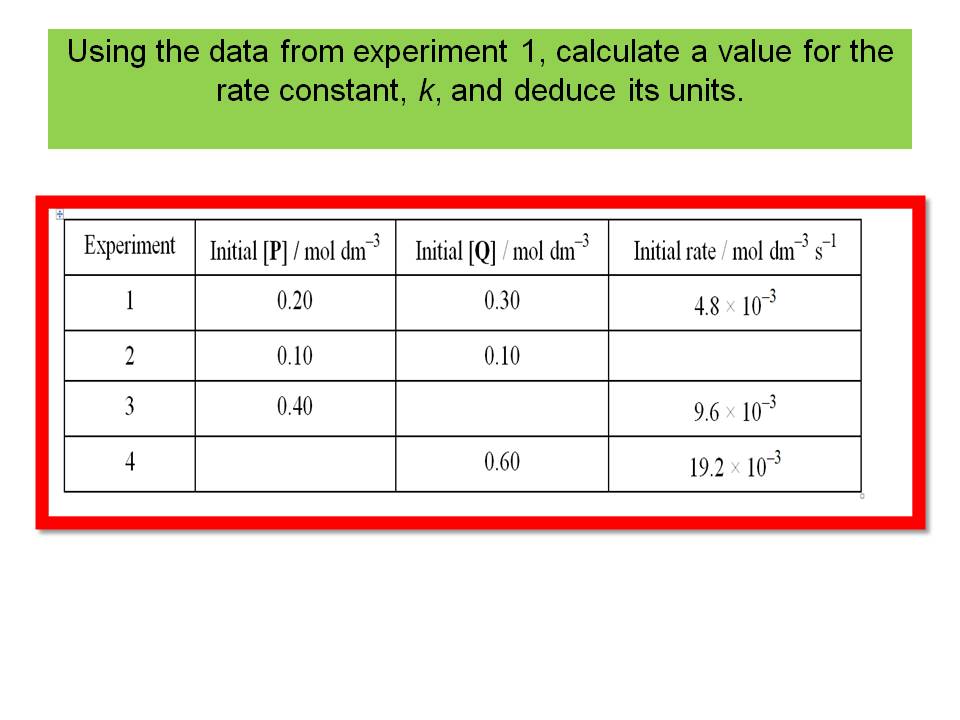
b.Using the data from experiment 1, calculate a value for the rate constant, k, and state its units.
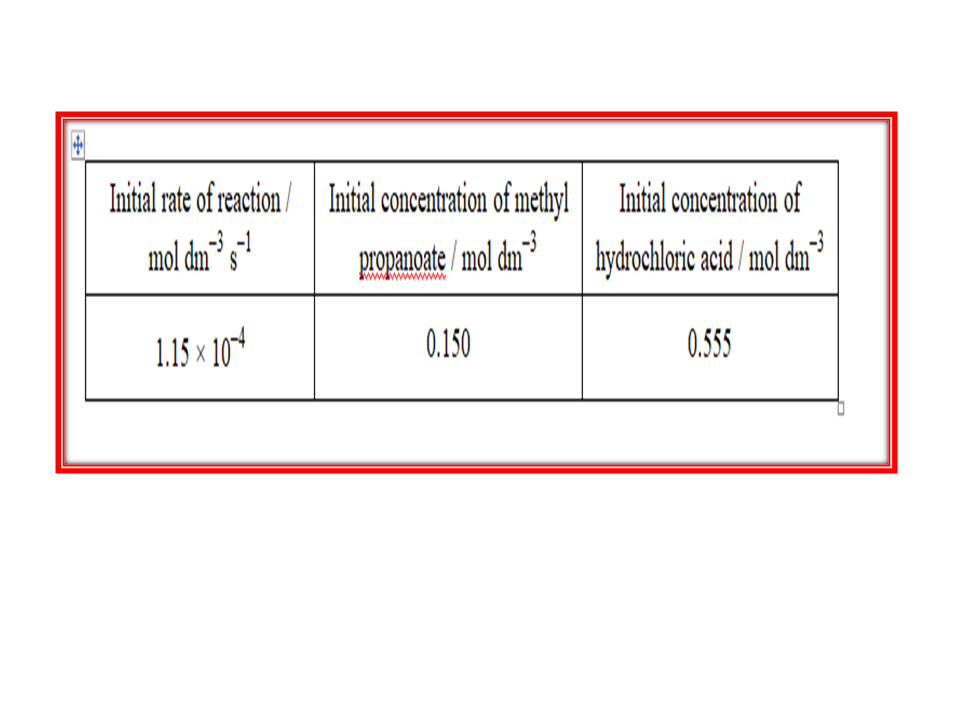
rate = k [CH3COCH3][H+]
•Use these data to calculate a value for the rate constant and deduce its units.



























































































































































































































































No comments:
Post a Comment